Provide the best quality
Ensure Patient Safety with Consistent Vaccine Storage & Drug Stability
Customers expect products to be of the highest quality, and when you’re dealing with cold storage for pharmaceuticals or pharmaceutical transport, consistent quality and safety is mandatory. If you can’t prove compliance, regulators may require the disposal of all drugs and vaccines. A reliable pharmaceutical temperature monitoring system from R&D to production is crucial in protecting your assets, your bottom line, and your patients.

Dickson Covers Your Monitoring Needs:
Delivering Solutions to Industry Leaders








Putting Your Customers First
When you choose the right monitoring system for your pharmaceuticals, you are putting the safety of patients first through the high quality of the products you produce. Out-of-range temperatures may affect the chemical stability of drugs and alter their physical properties, and present a potential danger to patients.

Supporting the Pharmaceutical Process
Whether you’re researching and producing a life-saving cure or managing pharmaceutical transport and storage for consumer-ready products, your work matters. Choosing the right solution for pharmaceutical monitoring is critical to your success—because at every point, you need consistency in your environmental conditions.

Download Our Pharmaceutical Temperature Monitoring Handbook
Our handbook explores the challenges and trends impacting pharmaceutical manufacturing, reviews core regulations, and discusses how monitoring can help your company address compliance challenges, stay audit-ready, and protect patients.
We understand your needs
Why Choose Dickson for Pharmaceutical Monitoring?
At Dickson, we understand the nuances of the pharmaceutical production process. From research to production to delivery, we know the importance of real-time, accurate data throughout the life cycle of your products. Our solutions are designed to remotely monitor essential environmental data throughout the pharmaceutical ecosystem so that your often irreplaceable assets, and your patients, stay protected.
Ensure Drug Quality & Safety
It’s difficult to tell the difference between a compromised product and a safe one. That is why continuous pharmaceutical temperature monitoring with real-time alerts is crucial. As a pharmaceutical provider, make sure that your products are never compromised and that you have documentation to prove it.
Prevent Product Loss
Have visibility into any excursions and automatically alert staff so they can immediately take corrective actions to prevent the loss of valuable drugs, vaccines, or lab samples.
- System-wide environmental monitoring
- Automatic data collection
- Real-time notification
Ensure Compliance
Easily comply with the pharmaceutical industry’s CGMP (Current Good Manufacturing Practice), GDP (Good Distribution Practices), and regulatory boards including the FDA, CDC, and local state Boards of Pharmacy. Reduce the risk of non-compliance with continuous monitoring and digital logs.
Delivering Solutions for Industry Leaders

Reliable & Safe Solutions
Dickson Serves All Areas in Pharma
From pharmaceutical manufacturing to vaccine storage and everything in between, Dickson covers all pharmaceutical temperature monitoring and compliance needs. In highly-regulated industries like pharma, there is a need for accurate and reliable environmental monitoring tools to ensure patient safety and guard against product loss. Stay on top of every environmental monitoring point throughout your pharmaceutical cold chain and remain compliant.
The best solutions
Pharmaceutical-Related Products
Dickson offers a broad portfolio of data loggers, sensors, and software tailored to meet the specific environmental monitoring requirements in the pharmaceutical industry. Automate the collection of temperature, humidity, and other environmental data with real-time notification, alarms, and customizable views using our products.
DicksonOne Display Logger
The DicksonOne Display Logger collects temperature, humidity, and differential pressure data, automatically delivering it to the DicksonOne cloud application. From there, you can securely access your data from any internet-connected device, anywhere in the world.
- WiFi/Ethernet
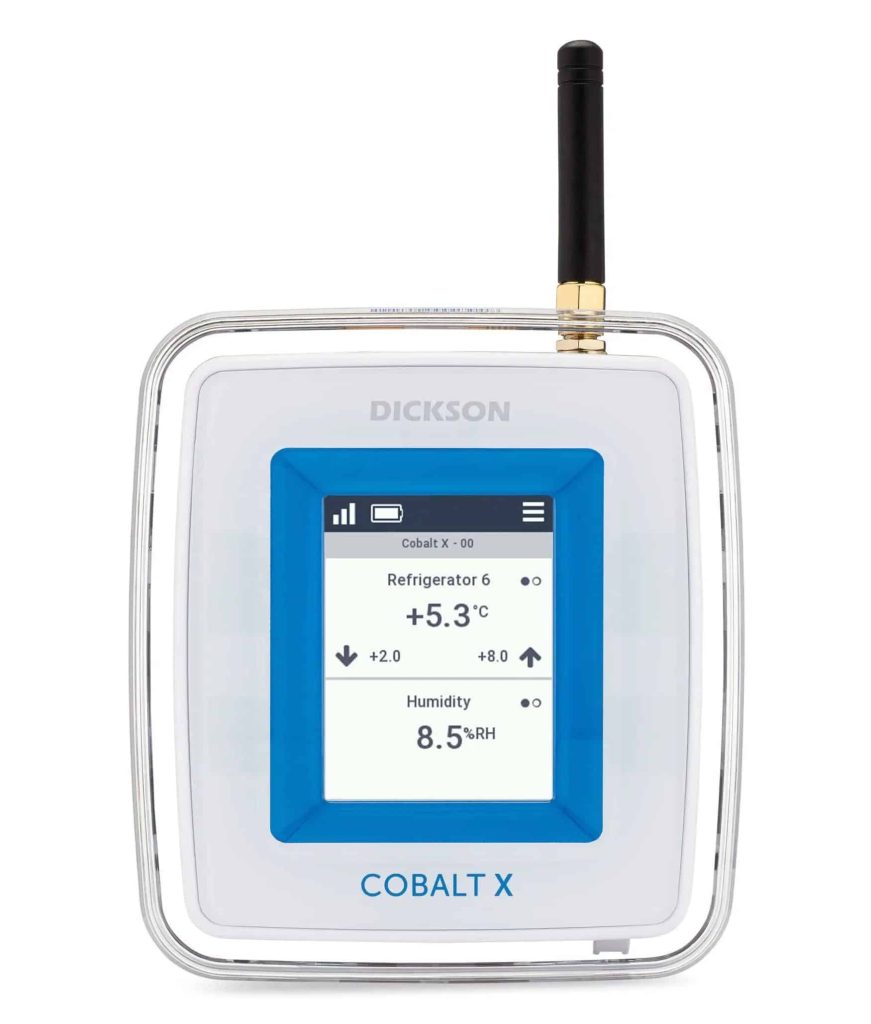
Cobalt X Data Logger
Cobalt X data loggers support up to 4 external sensors simultaneously to monitor your invaluable equipment. Readings are recorded in onboard memory, then transmitted to the OCEAView remote monitoring solution via LoRaWAN or Bluetooth Low Energy connectivity. The Cobalt X offers interactive touchscreen controls and highly visible alert indicators, with support for a wide variety of environmental sensors, including our Bluetooth-enabled remote sensors.
Battery-Operated Data Logger
With no AC power or WiFi required, our newest data logger allows you to monitor even the hardest to reach places. Thanks to impressive battery life, LoRa data transmission, and its compact size, the Battery-Operated Data Logger can go where no logger has gone before.
DicksonOne Touchscreen
A capacitive 8″ touchscreen offers our best user experience, featuring customizable views, alarms, and more. Plus, with cloud-based DicksonOne, you can access your data at your fingertips and anywhere you need it. Equipped with WiFi/Ethernet options and AC power for seamless integration into your system.
- WiFi & Ethernet
- AC Power
Reliable & Safe Solutions
Vaccines For Children (VFC)
Dickson offers a variety of services and product lines for VFC providers. Our CDC and VFC-compliant temperature monitoring system, DicksonOne, gives you total peace of mind with 24/7, real-time notifications.
Vaccine Storage and Handling Resources from the CDC
List of resources from the CDC:
Recommendations and Guidelines (CDC.gov)
Vaccine Storage and Handling Toolkit (PDF)
Vaccine Administration (CDC.gov)
Current Vaccine Shortages and Delays (CDC.gov)
Established in 1994, the Vaccines for Children (VFC) Program is a federally funded program, overseen by the Advisory Committee on Immunization Practices (ACIP) and the Center for Disease Control (CDC), created to make vaccines available for children whose families can’t afford them.
In order to stay eligible for this program, VFC providers need to meet strict regulations for vaccine storage, documentation, and record-keeping. VFC providers are required by the CDC to use digital data loggers or continuous temperature monitoring devices in vaccine storage units and transport units. To demonstrate compliance, accurate and reliable data is critical.
With Dickson’s VFC product bundles, you get VFC-compliant monitoring solutions to protect your vaccines which come ready with our glycol thermistor sensor and 1-Point NIST calibration making it easy to comply with VFC requirements.
With our digital data loggers powered by DicksonOne - our cloud-based environmental monitoring system - you can monitor your environment from anywhere, 24/7. This gives you peace of mind and ensures continuous monitoring of critical vaccines, record-keeping, and notification for temperature excursions.
Ready to chat?
Talk to a Specialist About Your Pharmaceutical Needs
Our trained team of professionals is ready to help you through the compliance process from start to finish. Reach out today to start the conversation. Call +1 (630) 543-3747 or send us a message.